Synthesis and characterization of poly-3-((2,5-hydroquinone)vinyl)-1H-pyrrole: investigation on backbone/pendant interactions in a conducting redox polymer†
Abstract
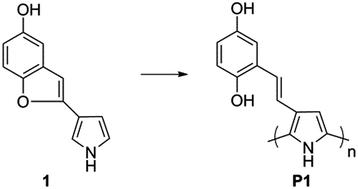
We herein report the synthesis and electrochemical characterization of poly-3-((2,5-hydroquinone)vinyl)-1H-pyrrole, consisting of a polypyrrole backbone derivatized at the beta position by a vinyl-hydroquinone pendant group. The structure of the polymer was characterized by solid state NMR spectroscopy. The interactions between the polypyrrole backbone and the oxidized quinone or reduced hydroquinone pendant groups are probed by several in situ methods. In situ attenuated total reflectance-Fourier transform infrared spectroscopy shows a spectroscopic response from both the doping of the polymer backbone and the redox activity of the pendant groups. Using an in situ Electrochemical Quartz Crystal Microbalance we reveal that the polymer doping is unaffected by the pendant group redox chemistry, as opposed to previous reports. Despite the continuous doping the electrochemical conversion from the hydroquinone state to the quinone state results in a significant conductance drop, as observed by in situ conductivity measurements using an Interdigitated Array electrode set-up. Twisting of the conducting polymer backbone as a result of a decreased separation between pendant groups due to π–π stacking in the oxidized state is suggested as the cause of this conductance drop.


 Please wait while we load your content...
Please wait while we load your content...