Tailoring the Schiff base photoswitching – a non-adiabatic molecular dynamics study of substituent effect on excited state proton transfer†
Abstract
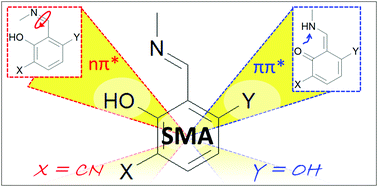
Small molecular systems exhibiting Excited State Intramolecular Proton Transfer (ESIPT) attract considerable attention due to their possible role as ultrafast, efficient, and photostable molecular photoswitches. Here, by means of static potential energy profile scan and on-the-fly non-adiabatic dynamics simulations we study the photodeactivation process of a minimal-chromophore aromatic Schiff base, salicylidene methylamine (SMA), and its two derivatives 6-cyano-salicylidene methylamine (6-CN-SMA) and 3-hydroxy-salicylidene methylamine (3-OH-SMA). We show that the dominant character of the lowest excited singlet state – ππ* vs. nπ* – plays a crucial role in the system's photophysics and controls the ESIPT efficiency. We also show that the relative alignment of the ππ* and nπ* states may be controlled through chemical substitutions made to the aromatic ring of the Schiff-base molecule. We believe that our findings will improve the rational-design strategies employed for the ESIPT systems, especially in the context of their possible photoswitching.


 Please wait while we load your content...
Please wait while we load your content...