Formulating the bonding contribution equation in heterogeneous catalysis: a quantitative description between the surface structure and adsorption energy†
Abstract
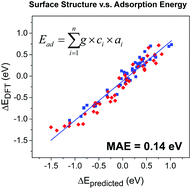
The relation between the surface structure and adsorption energy of adsorbates is of great importance in heterogeneous catalysis. Based on density functional theory calculations, we propose an explicit equation with three chemically meaningful terms, namely the bonding contribution equation, to quantitatively account for the surface structures and the adsorption energies. Successful predictions of oxygen adsorption energies on complex alloy surfaces containing up to 4 components are demonstrated, and the generality of this equation is also tested using different surface sizes and other adsorbates. This work may not only offer a powerful tool to understand the structure–adsorption relation, but may also be used to inversely design novel catalysts.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...