Kinetic analysis of overlapping multistep thermal decomposition comprising exothermic and endothermic processes: thermolysis of ammonium dinitramide†
Abstract
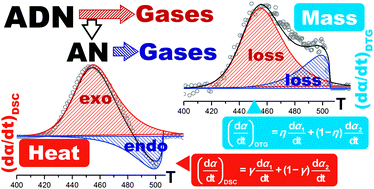
This study focused on kinetic modeling of a specific type of multistep heterogeneous reaction comprising exothermic and endothermic reaction steps, as exemplified by the practical kinetic analysis of the experimental kinetic curves for the thermal decomposition of molten ammonium dinitramide (ADN). It is known that the thermal decomposition of ADN occurs as a consecutive two step mass-loss process comprising the decomposition of ADN and subsequent evaporation/decomposition of in situ generated ammonium nitrate. These reaction steps provide exothermic and endothermic contributions, respectively, to the overall thermal effect. The overall reaction process was deconvoluted into two reaction steps using simultaneously recorded thermogravimetry and differential scanning calorimetry (TG–DSC) curves by considering the different physical meanings of the kinetic data derived from TG and DSC by P value analysis. The kinetic data thus separated into exothermic and endothermic reaction steps were kinetically characterized using kinetic computation methods including isoconversional method, combined kinetic analysis, and master plot method. The overall kinetic behavior was reproduced as the sum of the kinetic equations for each reaction step considering the contributions to the rate data derived from TG and DSC. During reproduction of the kinetic behavior, the kinetic parameters and contributions of each reaction step were optimized using kinetic deconvolution analysis. As a result, the thermal decomposition of ADN was successfully modeled as partially overlapping exothermic and endothermic reaction steps. The logic of the kinetic modeling was critically examined, and the practical usefulness of phenomenological modeling for the thermal decomposition of ADN was illustrated to demonstrate the validity of the methodology and its applicability to similar complex reaction processes.


 Please wait while we load your content...
Please wait while we load your content...