Intermolecular structure and hydrogen-bonding in liquid 1,2-propylene carbonate and 1,2-glycerol carbonate determined by neutron scattering†
Abstract
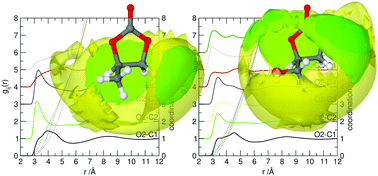
Neutron diffraction with isotopic substitution has been used to investigate the liquid structures of propylene carbonate and glycerol carbonate. C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen-bonding motifs dominate the local structure of propylene carbonate, giving rise to the formation of head-to-tail correlated chains of molecules. In contrast, glycerol carbonate exhibits a more disordered structure with no overall dominant interactions in which the pendant hydroxyl function disrupts structure-making correlations present in propylene carbonate.
C hydrogen-bonding motifs dominate the local structure of propylene carbonate, giving rise to the formation of head-to-tail correlated chains of molecules. In contrast, glycerol carbonate exhibits a more disordered structure with no overall dominant interactions in which the pendant hydroxyl function disrupts structure-making correlations present in propylene carbonate.


 Please wait while we load your content...
Please wait while we load your content...