Organic sensitizers featuring thiophene derivative based donors with improved stability and photovoltaic performance†
Abstract
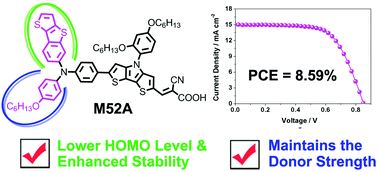
Thiophene derivatives, including thieno[3,2-b][1]benzothiophene (TBT), benzo[b]thiophene (BT), 2-phenylthieno[3,2-b]thiophene (PTT) and 2-phenylthiophene (PT), have been introduced as donors for the construction of triarylamine organic dyes (M52, M53, M56, M57 and M52A). The absorption, electrochemical and photovoltaic properties as well as the stabilities of these dyes are systematically investigated and compared with the reference dye (M55), whose donor is composed of the hexyloxybenzene (HOB) unit. It is found that introducing the TBT, BT, PTT or PT donors positively shifted the HOMO and LUMO levels of the organic dyes, providing a larger driving force for regeneration and reducing the energy loss for electron injection. In addition, we found that M52, which contains the TBT unit, exhibited better photovoltaic performance and photostability as compared to the reference dye. In contrast, M53 displayed the lowest efficiency and stability of these dyes, indicating that the BT unit is not a good building block for donors. Interestingly, upon the incorporation of the mixed donor (TBT–HOB), M52A achieved a desirable driving force for regeneration without a loss in light absorption, thus resulting in a further improved photovoltaic performance with respect to that of M52. This work demonstrates that introducing donors based on thiophene derivatives is a good strategy for tuning the energy levels and thereby enhancing the efficiency of the resulting devices.


 Please wait while we load your content...
Please wait while we load your content...