Jahn–Teller effect in LiMn2O4: influence on charge ordering, magnetoresistance and battery performance
Abstract
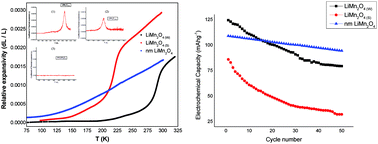
The phase transition near room temperature in LiMn2O4 was studied using thermal expansion measurements, and directly compared with the electrochemical performance of the material. Studies based on thermal expansion indicate the onset of a first-order phase transition at Tc ∼ 220 K for the nearly half-doped material, with [Mn3+]/[Mn4+] ≈ 1. The Tc shifts to a higher temperature, ∼290 K, and signatures for Verwey-type charge ordering at 290 K can be observed when the fraction of Jahn–Teller Mn3+ in LiMn2O4 is increased, i.e., [Mn3+]/[Mn4+] > 1. These studies show that the first-order phase transition near room temperature in LiMn2O4 is associated with charge ordering, which ultimately is a consequence of the Jahn–Teller effect. In addition, the Jahn–Teller effect is proven to be an important cause of magnetoresistance and electrochemical capacity fading in LiMn2O4. Electrochemical measurements show that both materials, either with a Tc ∼ 220 K or Tc ∼ 290 K, exhibit capacity fading to almost the same extent. Electrochemical capacity retention is observed only in nanosized LiMn2O4, for which the phase transition anomalies are completely absent.


 Please wait while we load your content...
Please wait while we load your content...