Controlling photophysics of styrylnaphthalimides through TICT, fluorescence and E,Z-photoisomerization interplay†
Abstract
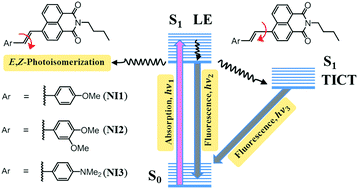
The photophysical properties of naphthalimide dyes NI1–3 with electron releasing 4-methoxy- (NI1), 3,4-dimethoxystyryl- (NI2) and dimethylaminostyryl (NI3) groups are examined in a variety of protic and aprotic solvents. All compounds demonstrate positive solvatochromism in the steady-state absorption and fluorescence spectra. The analysis of the dependence of the Stokes shift on the polarity of the solvent using the Lippert–Mataga equation allowed us to determine the change in the dipole moment upon excitation. The obtained data correspond to the formation of highly polar charge transfer states. Based on the transient absorption spectra and time-resolved fluorescence measurements, the presence of two different emissive states was definitely proved. The primarily formed planar Local Excited (LE) state dominates in non-polar solvents like cyclohexane and toluene where it relaxes mostly through fluorescence and E,Z-isomerisation pathways. In polar solvents, an alternative relaxation channel emerges that consists of twisting around single bond between styryl and naphthalimide fragments, which leads to the formation of a Twisted Intramolecular Charge Transfer (TICT) state. The factors affecting the fluorescence of TICT states are discussed. The observed spectral effects are rationalized using quantum-chemical calculations, X-ray data and NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...