In situ study of oxidation states of platinum nanoparticles on a polymer electrolyte fuel cell electrode by near ambient pressure hard X-ray photoelectron spectroscopy†
Abstract
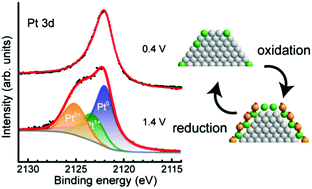
We performed in situ hard X-ray photoelectron spectroscopy (HAXPES) measurements of the electronic states of platinum nanoparticles on the cathode electrocatalyst of a polymer electrolyte fuel cell (PEFC) using a near ambient pressure (NAP) HAXPES instrument having an 8 keV excitation source. We successfully observed in situ NAP-HAXPES spectra of the Pt/C cathode catalysts of PEFCs under working conditions involving water, not only for the Pt 3d states with large photoionization cross-sections in the hard X-ray regime but also for the Pt 4f states and the valence band with small photoionization cross-sections. Thus, this setup allowed in situ observation of a variety of hard PEFC systems under operating conditions. The Pt 4f spectra of the Pt/C electrocatalysts in PEFCs clearly showed peaks originating from oxidized Pt(II) at 1.4 V, which unambiguously shows that Pt(IV) species do not exist on the Pt nanoparticles even at such large positive voltages. The water oxidation reaction might take place at that potential (the standard potential of 1.23 V versus a standard hydrogen electrode) but such a reaction should not lead to a buildup of detectable Pt(IV) species. The voltage-dependent NAP-HAXPES Pt 3d spectra revealed different behaviors with increasing voltage (0.6 → 1.0 V) compared with decreasing voltage (1.0 → 0.6 V), showing a clear hysteresis. Moreover, quantitative peak-fitting analysis showed that the fraction of non-metallic Pt species matched the ratio of the surface to total Pt atoms in the nanoparticles, which suggests that Pt oxidation only takes place at the surface of the Pt nanoparticles on the PEFC cathode, and the inner Pt atoms do not participate in the reaction. In the valence band spectra, the density of electronic states near the Fermi edge reduces with decreasing particle size, indicating an increase in the electrocatalytic activity. Additionally, a change in the valence band structure due to the oxidation of platinum atoms was also observed at large positive voltages. The developed apparatus is a valuable in situ tool for the investigation of the electronic states of PEFC electrocatalysts under working conditions.


 Please wait while we load your content...
Please wait while we load your content...