One-step hydroprocessing of fatty acids into renewable aromatic hydrocarbons over Ni/HZSM-5: insights into the major reaction pathways†
Abstract
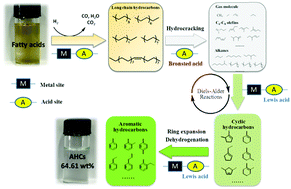
For high caloricity and stability in bio-aviation fuels, a certain content of aromatic hydrocarbons (AHCs, 8–25 wt%) is crucial. Fatty acids, obtained from waste or inedible oils, are a renewable and economic feedstock for AHC production. Considerable amounts of AHCs, up to 64.61 wt%, were produced through the one-step hydroprocessing of fatty acids over Ni/HZSM-5 catalysts. Hydrogenation, hydrocracking, and aromatization constituted the principal AHC formation processes. At a lower temperature, fatty acids were first hydrosaturated and then hydrodeoxygenated at metal sites to form long-chain hydrocarbons. Alternatively, the unsaturated fatty acids could be directly deoxygenated at acid sites without first being saturated. The long-chain hydrocarbons were cracked into gases such as ethane, propane, and C6–C8 olefins over the catalysts' Brønsted acid sites; these underwent Diels–Alder reactions on the catalysts' Lewis acid sites to form AHCs. C6–C8 olefins were determined as critical intermediates for AHC formation. As the Ni content in the catalyst increased, the Brønsted-acid site density was reduced due to coverage by the metal nanoparticles. Good performance was achieved with a loading of 10 wt% Ni, where the Ni nanoparticles exhibited a polyhedral morphology which exposed more active sites for aromatization.


 Please wait while we load your content...
Please wait while we load your content...