Conformational and electronic effects on the formation of anti cyclobutane pyrimidine dimers in G-quadruplex structures†
Abstract
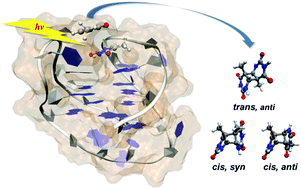
Cyclobutane pyrimidine dimers (CPDs) are the most commonly formed photochemical products when nucleic acids interact with UV radiation. In duplex DNA, the relative inflexible structure allows for only the cis, syn CPD isomer to be formed. G-quadruplex structures, however, have loops that are more flexible and allow for different orientations of the bases to interact. As a result, the highly unusual formation of an anti CPD has been observed in these structures. Due to the close proximity between two opposing loops containing the TTA sequence in two G-quadruplex structures (called “form-3” and “basket”), a high yield of anti CPD formation was expected in these structures. However, while significant yields of anti CPDs are observed in form-3, the anti CPD is hardly observed in the basket structure. To account for this inconsistency, we examine the process of anti CPD formation in form-3 and basket structures using simulations at the atomistic level. Here, we consider the conformational effect using MD simulations, which show whether the formation of the anti CPD is structurally feasible. Quantum mechanical/molecular mechanical (QM/MM) calculations of excited states are also used to consider the electronic effect by an adjacent guanine base which can quench the formation of the anti CPD through charge transfer (CT). Our results are in qualitative agreement with the experimental results, predicting a significant yield of the anti CPD in the form-3 structure and a negligible yield in the basket structure, while they also predict the formation of the cis, syn CPD between two opposing loops in form-3. Most importantly, our simulation results show that the yields of the anti CPD in the G-quadruplex are affected significantly by both conformational and electronic effects.


 Please wait while we load your content...
Please wait while we load your content...