One, two, and three-dimensional metal–organic coordination polymers derived from enantiopure organic phosphorate: homochirality, water stability and proton conduction†
Abstract
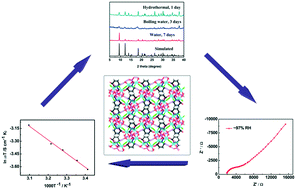
The solvothermal reaction of metal ions and D-H3pmpc yields three phosphonate-based metal–organic coordination polymers, {Cu(D-Hpmpc)(CH3OH)}n (PMOCP 1), {Cu(D-Hpmpc)}n (PMOCP 2) and {Cd2(D-pmpcH)(H2O)2Cl2}n (PMOCP 3) with diversified coordination fashions and dimensional features (D-H3pmpc = (D)-1-(phosphono-methyl)piperidine-3-carboxylic acid). PMOCP 1 exhibits a right-handed helical chain with a pitch of 6.785 Å and an interchain O–H⋯O hydrogen-bond. PMOCP 2 displays a chiral 2D network with a (4, 4) topology and a 1D O–H⋯O hydrogen-bond chain. PMOCP 3 shows a non-interpenetrating diamondoid architecture and various hydrogen-bond interactions. Their chiralities are verified by vibrational circular dichroism spectroscopy and second-order nonlinear optical response measurements. Compared with PMOCPs 1 and 2, PMOCP 3 has high water stability, a moderate proton conductivity of 1.38 × 10−4 S cm−1 at 323 K and ∼97% RH (relative humidity) and a lower activation energy of 0.14 eV.


 Please wait while we load your content...
Please wait while we load your content...