Synthesis and characterization of HPHT large single-crystal diamonds under the simultaneous influence of oxygen and hydrogen
Abstract
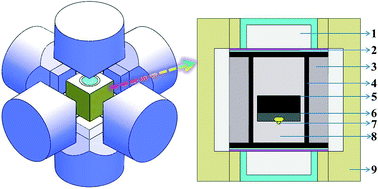
In this paper, we report the influence of oxygen and hydrogen additives in the metal melt on the growth process, morphology, and defect-and-impurity structure of large single-crystal diamonds. The main experiments were performed in the Ni70Mn25Co5–C system at pressure ranging from 5.5 GPa to 6.5 GPa, temperature from 1250 °C to 1550 °C and C6H8O7 concentration from 0 to 1.0 wt%. It was found that the synthesis conditions (temperature and pressure) increase with the increasing quantity of C6H8O7 additive. As the C6H8O7 content was increased from 0 to 0.8 wt%, the presence of oxygen and hydrogen was more likely to cause defects in the {100} surface. A further increase in the C6H8O7 concentration above approximately 0.9 wt% completely terminated the nucleation and growth of the diamonds and led to the crystallization of graphite alone. FTIR, Raman and XPS test results showed that oxygen and hydrogen coexist in the diamond crystal.


 Please wait while we load your content...
Please wait while we load your content...