Revealing the hydrothermal crystallization mechanism of ilmenite-type sodium niobate microplates: the roles of potassium ions†
Abstract
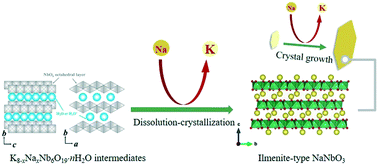
In this study, ilmenite-type NaNbO3 microplates were prepared by a facile surfactant-free method, using NaAc and KOH as a sodium resource and as a mineralizer, respectively. The effects of each reactant were identified, and the formation process and crystallization pathway of ilmenite-type NaNbO3 microplates were explored based on the phase constitution, microstructure and chemical compositional analysis. The indispensable roles of K ions, which participated in the formation of layered KNN-hydrate intermediates, were revealed. Ilmenite-type NaNbO3 microplates were formed by the dissolution of these intermediates and subsequent recrystallization into K-containing NaNbO3, followed by the ion-exchange between K and Na ions. Meanwhile excess K ions would hinder the release of K ions associated with the dissolution of intermediates, thus preventing the formation of ilmenite-type NaNbO3. Moreover, the morphological evolution of ilmenite-type NaNbO3 microplates from platelets to octahedra was interpreted in terms of their intrinsic structure and KOH concentration.


 Please wait while we load your content...
Please wait while we load your content...