Anion binding consistency by influence of aromatic meta-disubstitution of a simple urea receptor: regular entrapment of hydrated halide and oxyanion clusters†
Abstract
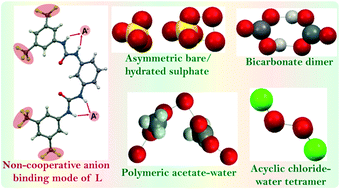
An electron-withdrawing meta-disubstituted trifluoro-methyl terminal containing bis-urea receptor L derived from meta-phenylenediamine core is logically designed and synthesized for investigating its anion coordination activities. Receptor L has been recognized as a potential system for unusual asymmetric entrapment of naked sulphate anion along with R33(5)-type cyclic hydrated sulphate by one of the three symmetry-independent receptor moieties in a unit cell via hydrogen bonding-activated proton transfer from hydrogen sulphate. In addition, it is effective for fluoride-induced fixation of atmospheric CO2 as an air-stable bicarbonate dimer, linear acetate–water polymeric assembly formation and chair-like (chloride)2–(water)2 assembly construction via H-bonding interactions of eight urea groups of four receptor units. Crystallographic results show that including DMF and DMSO-solvated free receptor structures, all halide and oxyanions noncovalently interact with urea groups of particular receptor moiety via noncooperative interactions irrespective of the anion dimension, which is possibly attributed to the aromatic meta-difunctionalization-driven steric effect of ligand architecture.


 Please wait while we load your content...
Please wait while we load your content...