Photoluminescence properties and energy transfer of Ce3+–Tb3+ co-doped SrAlF5 nanorods by a hydrothermal method†
Abstract
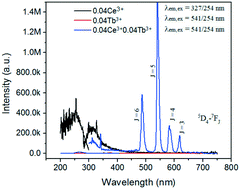
Ce3+ and Tb3+ co-doped SrAlF5 nanorods were synthesized via a mild hydrothermal method. The XRD results show that the prepared samples are single-phase. FE-SEM reveals the high uniformity of the as-synthesized nanorods (∼1 μm in length and ∼50 nm in diameter). The effect of reaction conditions on the morphology of SrAlF5 was studied. When excited at 254 nm, contrasting weak emission signals of SrAlF5:Tb3+ were observed, while SrAlF5:Ce3+,Tb3+ nanorods exhibited the strong green emission of Tb3+ ions. The energy transfer between Ce3+ and Tb3+ was observed and studied. The mechanism of energy transfer was deduced to be the electric dipole–dipole interaction in the SrAlF5 host. The optimum doping concentration of Ce3+ ions is 7 mol% in SrAlF5:0.01Tb3+. The fluorescence decay curves for Ce3+ and Tb3+ of SrAlF5:Ce3+,Tb3+ nanorods were measured at room temperature. The lifetime of Ce3+ is in the range from 78.6 to 1.2 ns and the lifetime of Tb3+ varies from 4.39 to 7.39 ms. The energy transfer efficiency from Ce3+ to Tb3+ was calculated using lifetime and emission spectra. The chromaticity coordinates of all the samples were calculated and the chromaticity coordinates of SrAlF5:0.06Ce3+,0.01Tb3+ are very near those of the European broadcasting union (EBU) primary green color (0.29, 0.60).


 Please wait while we load your content...
Please wait while we load your content...