Modulating the assembly of N-benzylideneaniline by halogen bonding: crystal, cocrystal and liquid crystals†
Abstract
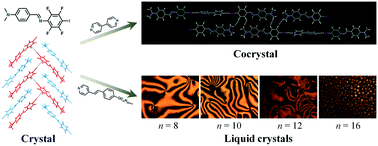
A novel halogen bonding (XB) donor–acceptor N-benzylideneaniline 3, containing one terminal of iodo-tetrafluorobenzene and the other side of 4-dimethylaminophenyl connected by a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, has been designed and synthesized. As a self-assembled halogen-bonded motif, 3 exists in two conformations and they are antiparallel to generate fishbone-like stacks by π⋯π and C–I⋯π intermolecular interactions. As a halogen-bonded donor, it can connect with 4,4′-bipyridine to constitute 2 : 1 ratio cocrystal with C–I⋯NPy interactions. When the acceptor changes to 4-alkoxystilbazole, liquid crystal property varies with the length of the terminal chains. DSC and POM experiments demonstrated a series of XB liquid crystals. Among them, 3-A10 shows a long range of nematic phase and an integrated schlieren texture.
N bond, has been designed and synthesized. As a self-assembled halogen-bonded motif, 3 exists in two conformations and they are antiparallel to generate fishbone-like stacks by π⋯π and C–I⋯π intermolecular interactions. As a halogen-bonded donor, it can connect with 4,4′-bipyridine to constitute 2 : 1 ratio cocrystal with C–I⋯NPy interactions. When the acceptor changes to 4-alkoxystilbazole, liquid crystal property varies with the length of the terminal chains. DSC and POM experiments demonstrated a series of XB liquid crystals. Among them, 3-A10 shows a long range of nematic phase and an integrated schlieren texture.


 Please wait while we load your content...
Please wait while we load your content...