Trimethyl-, triethyl- and trimethoxybenzene-based tripodal compounds bearing pyrazole groups: conformations and halogen-/hydrogen-bond patterns in the crystalline state†
Abstract
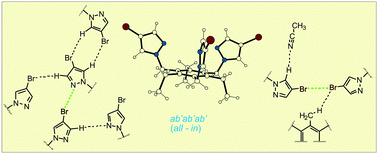
X-ray analyses of a series of benzene-based tripodal molecules 1–9, bearing either bromo-substituted pyrazole groups or analogues lacking the bromo substituents, provide interesting insights into the molecular recognition phenomena and also give information about the different conformations which adopt the tripodal molecules in the solvent-free crystals and in solvates. The crystal packings are characterized by the presence of C–H⋯O, C–H⋯N, C–H⋯Br and C–H⋯Cl hydrogen bonds as well as C–Br⋯N, C–Br⋯Br, C–Br⋯Cl and C–Br⋯π halogen bonds. The conformations observed in the case of the triethyl- and trimethoxybenzene-based compounds can be defined as ab'ab'ab', ab'ab'ba', ab'ab'bb', ab'aa'ba', ab'aa'bb', aa'ab'ba' and aa'aa'aa' with the pyrazole-bearing arms arranged either in an aaa or an aab fashion.


 Please wait while we load your content...
Please wait while we load your content...