Enantiospecific recognition of 2-butanol by an inherently chiral cavitand in the solid state†
Abstract
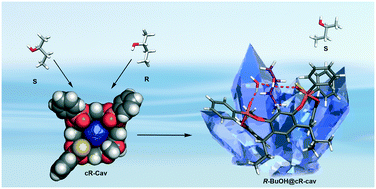
A new inherently chiral cavitand is described, in which the desymmetrization of the rigid concave cavity is achieved by introducing three different bridging groups at the upper rim, namely two inward P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, one inward P
O groups, one inward P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moiety and one methylene bridge in the AABC mode. The racemic mixture of cR and cS enantiomers is resolved by semi-preparative chiral HPLC and the enantiopurity confirmed through circular dichroism. The properties of the enantiopure cR-cav in the enantioselective recognition of racemic 2-butanol is studied by co-crystallization experiments. The exclusive formation of the complex R-BuOH@cR-cav demonstrates that cR-cav is able to discriminate between the two enantiomers of the alcohol in the solid state. The enantiospecific recognition exhibited by the cR-cav towards racemic 2-butanol is particularly relevant because of the low degree of chirality of the alcohol (1.9 calculated by CCM algorithm).
S moiety and one methylene bridge in the AABC mode. The racemic mixture of cR and cS enantiomers is resolved by semi-preparative chiral HPLC and the enantiopurity confirmed through circular dichroism. The properties of the enantiopure cR-cav in the enantioselective recognition of racemic 2-butanol is studied by co-crystallization experiments. The exclusive formation of the complex R-BuOH@cR-cav demonstrates that cR-cav is able to discriminate between the two enantiomers of the alcohol in the solid state. The enantiospecific recognition exhibited by the cR-cav towards racemic 2-butanol is particularly relevant because of the low degree of chirality of the alcohol (1.9 calculated by CCM algorithm).


 Please wait while we load your content...
Please wait while we load your content...