Two-component organic crystals without hydrogen bonding: structure and intermolecular interactions in bimolecular stacking†
Abstract
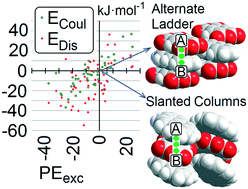
A survey of crystal structures including two organic compounds unable to form hydrogen bonding has been carried out using the Cambridge Structural Database. Such systems are common and numerous. Association modes mostly include stacking of flat systems, one of them usually being an aromatic hydrocarbon. “Alternate-ladder” (AL) and “slanted column” (SC) motifs occur most frequently; AL is somewhat prevalent in fluoroarene and pyromellitic dianhydride cocrystals, whereas SC occurs preferentially, but not exclusively, with quinones, nitrobenzene, TCNB and TCNQ coformers. Segregation of A and B chemical units in separate columns is very seldom observed, while A⋯B is the energetically dominating pair in a vast majority of the cases. A stable network of stacked A⋯B units seems to be a strict requirement for observing cocrystallization, even more than an overall higher stability of the cocrystal with respect to its coformers. This highlights the central role of kinetic factors in determining the drive to stacking hetero-recognition. Energy dissection using the PIXEL scheme shows that dispersion energies play a dominant but not exclusive role. The interaction between flat organic systems is found to be a viable synthetic approach for cocrystallization, on the same footing as the more popular O–H⋯O, O–H⋯N and N–H⋯O hydrogen bonding. Some practical suggestions for the choice of the best coformers are provided.


 Please wait while we load your content...
Please wait while we load your content...