Low temperature surfactant-free synthesis of monodisperse β-NaGdF4 nanorods by a novel ion-exchange process and their luminescence properties†
Abstract
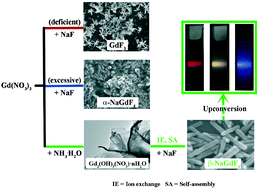
In this paper, a rationally designed low temperature and surfactant-free ion-exchange strategy to synthesize monodisperse hexagonal NaGdF4 nanorods from a layered gadolinium hydroxynitrate precursor is reported for the first time. This novel synthetic process takes advantage of the structural similarity between the precursor and the hexagonal NaGdF4 to avoid the formation of the kinetically favored cubic phase. The formation mechanisms were discussed in detail by tracking the phase and morphology evolution during the reaction process. In addition, the downconversion and upconversion properties were investigated under laser excitation, and the products exhibited intense characteristic luminescence.


 Please wait while we load your content...
Please wait while we load your content...