Crystallisation of a salt hydrate with a complex solid form landscape†
Abstract
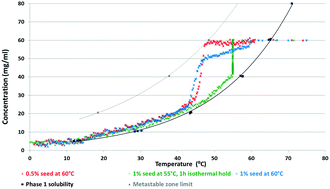
Different approaches were utilized to assess the thermodynamic stability relationship between four distinct hemihydrates of sitagliptin L-tartrate (SLT). These phases are true polymorphs, having the same chemical composition and molar ratio of water, but different arrangement in the crystal lattice. The general approaches (Burger and Ramberger rules) have only limited applicability, but indicated a monotropic relationship between Phase 1 and Phase 2. The crystallisation and transformation behaviour of the polymorphs was strongly influenced by the solvent composition. As the stability thereof was found to be solvent-dependent, the application of the van't Hoff plot or the solvent-mediated transformation did not unambiguously reveal the relative stability of the forms. Experiments were carried out in an endeavour to determine the most suitable concentration trajectory for cooling crystallisation of the selected candidate and prevent concomitant crystallisation. The application of in situ monitoring techniques, such as FTIR-ATR and FBRM, offered great potential for process optimization and control.


 Please wait while we load your content...
Please wait while we load your content...