Halogen bonding modulates hydrogel formation from Fmoc amino acids†
Abstract
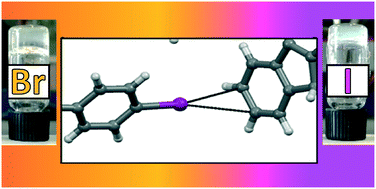
The single crystal X-ray structures of a series of p-halogenated Fmoc phenylalanines highlight the role of halogen bonding in their supramolecular organization in the solid state. The amino acids showing the occurrence of halogen bonding resulted in gels with the strongest mechanical properties. All gelation parameters could be ranked according to halogen atom polarizability, i.e., halogen bond strength.


 Please wait while we load your content...
Please wait while we load your content...