Fenamic acid crystal with two asymmetric units (Z′ = 2): why Z′ = 2 rather than Z′ = 1†
Abstract
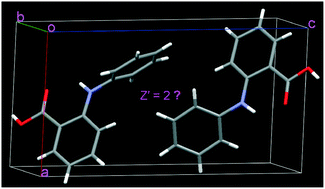
Two molecules with different conformations appear in the asymmetric unit of fenamic acid crystal (Z′ = 2). The two conformations have higher energies, and they dimerize via asymmetric O–H⋯O hydrogen bonds. For the predicted crystal (Z′ = 1), the symmetric O–H⋯O hydrogen bonds form between the lower energy conformers. Quantum mechanical calculations were carried out to elaborate the existence of higher energy conformers and their special arrangements. The asymmetric O–H⋯O hydrogen bonds in the crystal (Z′ = 2) are even stronger than the symmetric O–H⋯O hydrogen bonds in the predicted crystal (Z′ = 1), which is an important reason that the crystal with Z′ = 2 is observed. Additionally, intricate intermolecular interactions, that is, many adjacent molecules interacting with the substituted and unsubstituted benzene groups of higher energy conformers, also come into play, and these interactions cooperate and stabilize the conformers. A comparison of these intermolecular interactions in the crystal (Z′ = 2) with those in the predicted crystal (Z′ = 1) was made. The potential factors holding the two higher energy conformers together as a packing motif have been discussed in detail. We expect that the results could shed light on the understanding of the origin of the Z′ = 2 structure and other higher Z′ structures.


 Please wait while we load your content...
Please wait while we load your content...