Nature of the 550 nm emission and d0 ferromagnetism in ZnO nanocrystals revealed by H2O2 etching†
Abstract
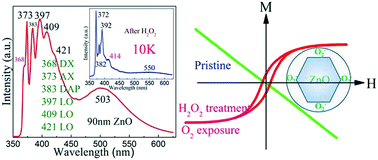
Clarifying the origin of green photoluminescence and the room temperature ferromagnetism in ZnO is crucial for its potential application. In this work, we show that the characteristic 368 nm donor-bound exciton and the g = 1.96 electron spin resonance (ESR) signal in ZnO nanocrystals were eliminated after H2O2 oxidation, along with the appearance of 550 nm photoluminescence, unambiguously evidencing the creation of acceptor defects. Meanwhile, the d0 ferromagnetism was significantly enhanced. In combination with the fact that ambient O2 exposure also resulted in a significant boost of ferromagnetism in ZnO particles with different grain sizes, we surmise that adsorbed oxygen facilitates the d0 ferromagnetism in ZnO while VZn causes the 550 nm green emission. This work illustrates that oxygen exposure is an efficient way to tune the physics in metal oxides through engineering defects.


 Please wait while we load your content...
Please wait while we load your content...