Interplay between structure and magnetism in the low-dimensional spin system: K(C8H16O4)2CuCl3·H2O†
Abstract
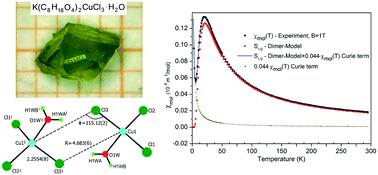
Materials based on a crown ether complex together with magnetic ions, especially Cu(II), can be used to synthesize new low dimensional quantum spin systems. We have prepared the new crown ether complex di-μ-chloro-bis(12-crown-4)-aquadichloro-copper(II)-potassium, K(C8H16O4)2CuCl3·H2O (1), determined its structure, and analyzed its magnetic properties. Complex (1) has a monoclinic structure and crystallizes in the space group P21/n with lattice parameters of a = 9.5976(5) Å, b = 11.9814(9) Å, c = 21.8713(11) Å and β = 100.945(4)°. The magnetic properties of this compound have been investigated in the temperature range 1.8–300 K. The magnetic susceptibility shows a maximum at 23 K, but no 3-D long range magnetic order down to 1.8 K. The S = 1/2 Cu(II) ions form antiferromagnetically coupled dimers with Cu–Cl distances of 2.2554(8) Å and 4.683(6) Å, and a Cu–Cl–Cu angle of 115.12(2)° with 2Jdimer = −2.96 meV (−23.78 cm−1). The influence of H2O on the Cl–Cu–Cl exchange path is analyzed. Our results show that the values of the singlet–triplet splitting are increasing considering H2O molecules in the bridging interaction. This is supported by density functional theory (DFT) calculations of coupling constants with Perdew and Wang (PWC), and Perdew, Burke and Ernzerhof (PBE) and the strongly constrained and appropriately normed (SCAN) exchange-correlation function show excellent agreement for the studied compound.


 Please wait while we load your content...
Please wait while we load your content...