Effect of ferrous iron on the nucleation and growth of CaCO3 in slightly basic aqueous solutions†
Abstract
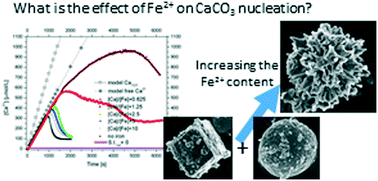
The precipitation of calcium carbonate, CaCO3 and the growth of calcite has been studied in aqueous solutions containing ferrous iron (Fe2+). Two different types of bulk experiments have been carried out: nucleation experiments at constant pH, following the procedure developed by Gebauer et al. (2008), revealed the stabilisation of aragonite induced by the presence of the foreign ions at the expense of calcite and vaterite. Growth experiments, using the constant composition method (Tomson and Nancollas, 1978), clearly showed that calcite growth is inhibited by the presence of Fe2+ and the characterisation of the re-grown calcite crystals by HR-TEM confirmed the predictions obtained with molecular dynamics simulations. Additionally, in situ atomic force microscopy (AFM) flow-through growth experiments directly showed the distortion of the normal calcite growth spirals that lead to macroscopic inhibition of calcite growth. Finally, thermodynamic considerations for the solid solution – aqueous solution system Ca–Fe–CO2–H2O are discussed that allow the modelling of geochemical processes involved in this system, such as geological carbon storage in basaltic rocks.


 Please wait while we load your content...
Please wait while we load your content...