Aromaticity switching via azulene transformations in azulene-bridged A,D-dithiahexaphyrin†
Abstract
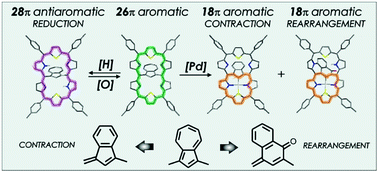
The incorporation of an azulene bridge into an aromatic hexaphyrin framework allows manipulating π-electron delocalization pathways. The palladium(II) complex undergoes hydroxyl-triggered azulene contraction or isomerization to an oxynaphthalene unit, transforming the hexaphyrin framework into meso-linked carbaporphyrins. This converts the 26π-electron pathway into the 18π one.


 Please wait while we load your content...
Please wait while we load your content...