Modular synthesis of 4-aminocarbonyl substituted 1,8-naphthalimides and application in single molecule fluorescence detection†
Abstract
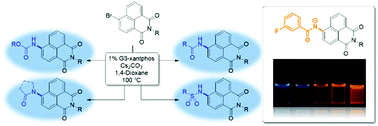
Robust methodology to install amide, carbamate, urea and sulfonamide functionality to the 1,8-naphthalimide scaffold has been developed and exemplified. New benzamidonaphthalimide 6, synthesised using this approach, was found to be sensitive to base whereupon fluorescence emission strongly increases (>10-fold) and red-shifts (>4000 cm−1). The optical properties of deprotonated 6 allow for single molecule fluorescence detection, the first example of such behaviour from this class of fluorophore.
- This article is part of the themed collection: Chemosensors and Molecular Logic


 Please wait while we load your content...
Please wait while we load your content...