Guest control of a hydrogen bond-catalysed molecular rotor†
Abstract
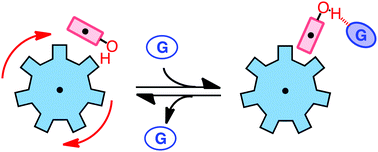
Herein, the control of a molecular rotor using hydrogen bonding guests is demonstrated. With a properly positioned phenol substituent, the N-arylimide rotors can form an intramolecular hydrogen bond that catalyses the rotational isomerization process. The addition of the guests disrupts the hydrogen bond and raises the rotational barrier, slowing the rotation by two orders of magnitude.


 Please wait while we load your content...
Please wait while we load your content...