N-Heterocyclic carbene-induced transmethylation in tungsten imido alkylidene bistriflates: unexpected formation of an N-heterocyclic olefin complex†
Abstract
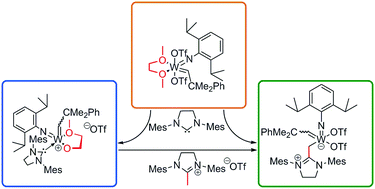
The reaction of [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(OTf)2(DME)] (DME = 1,2-dimethoxyethane, OTf = CF3SO3) with the N-heterocyclic carbene (NHC) 1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene (IMesH2) leads to DME activation followed by transmethylation and in due consequence to the formation of the N-heterocyclic olefin complex [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(OTf)2(IMesH2CH2)] along with [W(N-2,6-iPr2C6H3)(CHCMe2Ph)(OTf)(IMesH2)(κ2-O(CH2)2OMe)], [1,3-bis(2,4,6-trimethylphenyl)-2-methylimidazolidinium+ (OTf−)] and [1,3-bis(2,4,6-trimethylphenyl)-2-H-imidazolidinium+ (OTf−)]. A reaction pathway is proposed and confirmed by the use of 13C-labelled compounds; structures of the products were verified by NMR and/or single-crystal X-ray analysis.


 Please wait while we load your content...
Please wait while we load your content...