Abstract
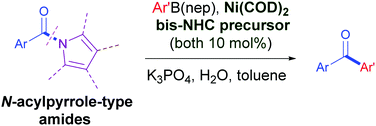
The catalytic conversion of amides to ketones is highly desirable yet challenging in organic synthesis. We herein report the first Ni/bis-NHC-catalyzed cross-coupling of N-acylpyrrole-type amides with arylboronic esters to obtain diarylketones. This method is facilitated by a new chelating bis-NHC ligand. The reaction tolerates diverse functional groups on both arylamide and arylboronic ester partners including sensitive ester and ketone groups.


 Please wait while we load your content...
Please wait while we load your content...