Organocatalysis using aldehydes: the development and improvement of catalytic hydroaminations, hydrations and hydrolyses
Abstract
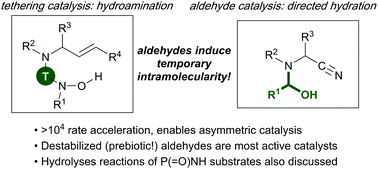
Organocatalysis has emerged as a powerful approach to facilitate and accelerate various difficult reactions. This Feature article presents recent developments and improvements using aldehydes as catalysts in difficult Cope-type intermolecular hydroamination, hydration and hydrolysis reactions. Most reactions exploit temporary intramolecularity. In catalytic Cope-type hydroaminations of allylic amines, aldehydes act as tethering catalysts, and allow room temperature reactions and high enantio- or diastereoselectivities if chiral aldehydes or reagents are used. Mechanistic studies showed that simpler catalysts such as formaldehyde are more active due to an improved ability to form the temporary tether, which translated in an improved reaction scope. Gratifyingly, improved catalytic efficiency and broad reaction scope were also observed in the aldehyde-catalyzed hydration of α-amino nitriles. Since destabilized aldehydes often favor temporary intramolecularity, this led to a comparison of the catalytic activity of several carbohydrates, and to experiments relevant in the prebiotic “origin of life” chemistry context. Studies on catalytic hydrolysis reactions of organophosphorous reagents are also presented, in which o-phthalaldehyde performs electrophilic activation of phosphinic amides, and other substrates possessing the P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH motif. Overall, this Feature article shows that aldehydes can be efficient catalysts in a variety of reactions, and highlights the efficiency of destabilized aldehydes such as formaldehyde and simple carbohydrates in this context.
O)NH motif. Overall, this Feature article shows that aldehydes can be efficient catalysts in a variety of reactions, and highlights the efficiency of destabilized aldehydes such as formaldehyde and simple carbohydrates in this context.


 Please wait while we load your content...
Please wait while we load your content...