Effect of protic additives in Cu-catalysed asymmetric Diels–Alder cycloadditions of doubly activated dienophiles: towards the synthesis of magellanine-type Lycopodium alkaloids†
Abstract
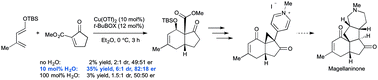
The pronounced beneficial effect of a precise amount of protic additive in an enantioselective Cu-catalysed Diels–Alder reaction is reported. This reaction, which employs a cyclic alkylidene β-ketoester as a dienophile, represents one of the first examples of a transformation where these extremely versatile, though highly unstable reaction partners participate effectively in catalytic asymmetric cycloaddition with a functionalised diene. The cycloadduct was used as an intermediate towards the synthesis of magellanine-type Lycopodium alkaloids featuring a Stille cross-coupling of a highly congested enol triflate and a unique Meinwald rearrangement/cyclopropanation sequence.


 Please wait while we load your content...
Please wait while we load your content...