Tandem trifluoromethylthiolation/aryl migration of aryl alkynoates to trifluoromethylthiolated alkenes†
Abstract
A trifluoromethylthiolation initiated aryl migration of aryl alkynoates was disclosed. This protocol employs AgSCF3 as the SCF3 source and MeCN as both the solvent and the hydrogen source. This provides a new access to trifluoromethylthiolated alkenes from readily available substrates and reagents.
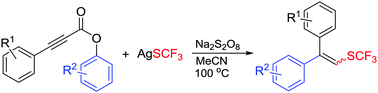


 Please wait while we load your content...
Please wait while we load your content...