2,2-Diiododimedone: a mild electrophilic iodinating agent for the selective synthesis of α-iodoketones from allylic alcohols†
Abstract
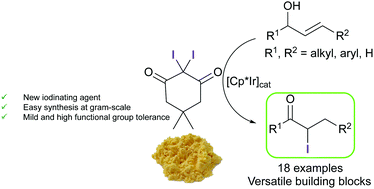
2,2-Diiodo-5,5-dimethylcyclohexane-1,3-dione is reported as a new electrophilic iodinating agent that selectively iodinates electron-rich aromatics. In contrast to other common electrophilic iodinating reagents, its mild nature allows it to be used for the selective synthesis of α-iodinated carbonyl compounds from allylic alcohols through a 1,3-hydrogen shift/iodination process catalyzed by iridium(III) complexes.


 Please wait while we load your content...
Please wait while we load your content...