Unusual reactivity of rhodium carbenes with allenes: an efficient asymmetric synthesis of methylenetetrahydropyran scaffolds†
Abstract
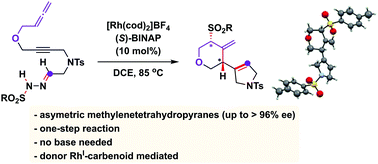
A RhI/(S)-BINAP catalytic system is able to promote carbene alkyne metathesis and cascade this elemental step with an stereoselective reaction with allenes. An unusual carbene/allene reactivity is discovered that, through a formal addition of p-toluenesulfinic acid to a Rh-bound trimethylenemethane intermediate, affords 4-methylenetetrahydropyran compounds in good yields and excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...