A CuI/CuIII prototypical organometallic mechanism for the deactivation of an active pincer-like CuI catalyst in Ullmann-type couplings†
Abstract
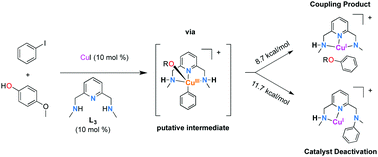
Unraveling the mechanistic details of copper-catalyzed arylation of nucleophiles (Ullmann-type couplings) is a very challenging task. It is a matter of intense debate whether it is a radical-based process or an organometallic redox-based process. The ancillary ligand choice in Ullmann-type couplings plays a key role in such transformations and can strongly influence the catalytic efficiency as well as the mechanism. Here, we show how a predesigned tridentate pincer-like catalyst undergoes a deactivation pathway through a CuI/CuIII prototypical mechanism as demonstrated by helium-tagging infrared photodissociation (IRPD) spectroscopy and DFT studies, lending a strong support to the existence of an aryl–CuIII species in the Ullmann couplings using this tridentate ligand.


 Please wait while we load your content...
Please wait while we load your content...