Iridium-catalysed hydrosilylation of cyclopropanes via regioselective carbon–carbon bond cleavage†
Abstract
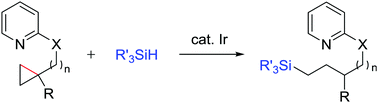
While cyclopropanes have been explored as synthetically valuable building blocks, their transformation without conjugated substituents or directly substituted heteroatoms remains challenging. The current study describes the iridium-catalysed ring-opening hydrosilylation of cyclopropanes. A nitrogen-based directing group was found to control the reactivity of iridium active species as well as the regiochemistry of carbon–carbon bond cleavage and hydrosilylation.


 Please wait while we load your content...
Please wait while we load your content...