Asymmetric tandem conjugate addition–protonation to forge chiral secondary C–O bonds for quaternary carbon stereocenters at the nonadjacent β-position†
Abstract
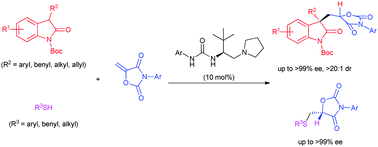
A direct strategy to construct chiral secondary C–O bonds for quaternary carbon stereocenters at the nonadjacent β-position is described. Methylene 1,3-oxazolidine-2,4-diones were for the first time employed in an asymmetric reaction as viable electrophiles undergoing a tandem conjugate addition–protonation process. Using an L-amino acid-based urea–tertiary amine catalyst, the reaction with 3-substituted oxindoles gave valuable protonation adducts featuring 1,3-quaternary–tertiary (C–O) nonadjacent stereocenters in high yields and excellent stereoselectivities. The method was successfully extended to sulphur nucleophiles for synthesizing chiral 1,3-sulfur-tertiary alcohol derivatives with bioactive importance.


 Please wait while we load your content...
Please wait while we load your content...