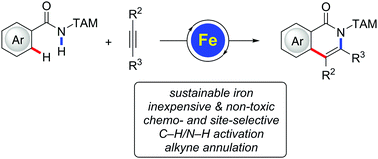
Iron-catalyzed C–H/N–H activation by triazole guidance: versatile alkyne annulation†
Abstract
Iron-catalyzed C–H/N–H functionalizations were achieved by the aid of modular triazole amides. The alkyne annulation allowed for the expedient synthesis of valuable isoquinolone scaffolds with high levels of chemo-, site- and regio-selectivities.


 Please wait while we load your content...
Please wait while we load your content...