A zwitterionic hydrocarbon-soluble borenium ion based on a β-diketiminate backbone†
Abstract
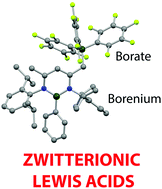
A versatile synthetic route has been developed to access strongly Lewis acidic borenium cations (and heavier group 13 analogues) featuring a pendant weakly-coordinating borate function. The hydrocarbon-soluble borenium/borate zwitterion is more strongly Lewis acidic than B(C6F5)3, despite featuring a pendant (non-fluorinated) aryl group and two flanking N-donors.


 Please wait while we load your content...
Please wait while we load your content...