Multifunctional catalysis: stereoselective construction of α-methylidene-γ-lactams via an amidation/Rauhut–Currier sequence†
Abstract
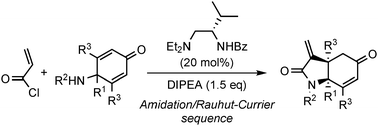
Mixing of acryloylchloride, dienone 2, N,N-diisopropylethylamine with chiral organocatalyst 5a, which could simultaneously act as Brønsted and Lewis base catalysts, led to a one-pot amidation/Rauhut–Currier sequence, affording α-methylidene-γ-lactams 4. Catalyst 5a could be recovered and reused by acid/base extraction without any loss of catalytic activity in the stepwise protocol.


 Please wait while we load your content...
Please wait while we load your content...