Palladium and visible-light mediated carbonylative Suzuki–Miyaura coupling of unactivated alkyl halides and aryl boronic acids†
Abstract
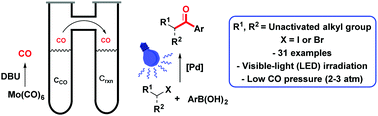
Herein, a simple and efficient method for the palladium-catalyzed carbonylation of aryl boronic acids with unactivated alkyl iodides and bromides under visible-light irradiation, ambient temperature and low CO-pressure is presented. Notably, the procedure uses readily available equipment and an inexpensive palladium catalyst to generate the key alkyl radical intermediate. These mild conditions enabled the synthesis of a range of functionalized aryl alkyl ketones including the antipsychotic drug, melperone.


 Please wait while we load your content...
Please wait while we load your content...