A square-planar nickel dithiolate complex as an efficient molecular catalyst for the electro- and photoreduction of protons†
Abstract
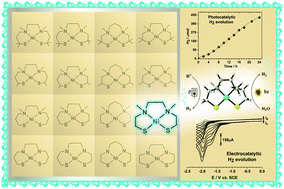
A square-planar nickel(II) dithiolate complex is an active molecular catalyst for both photoreduction of protons from water with a turnover number (>1500) and electroreduction of protons from weakly acidic solutions with remarkable turnover frequencies (5575 s−1 at −1.92 V and 1441 s−1 at −1.61 V vs. SCE). DFT calculations provide in-depth insight into the catalytic cycle of the electrochemical reaction, suggesting that the sulfur atoms play crucial roles in proton exchange and hydrogen formation.


 Please wait while we load your content...
Please wait while we load your content...