Kinetic resolution via supramolecular iminium catalysis: multiactivation enables the asymmetric synthesis of β-aryl substituted aldehydes and densely functionalized cyclohexanes†
Abstract
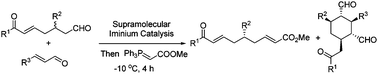
The kinetic resolution of β-aryl substituted 1,7-dioxo compounds with α,β-unsaturated aldehydes affording enantioenriched β-aryl substituted aldehydes as well as densely functionalized cyclohexanes is presented. The two enantioenriched products were obtained in reasonable yields with high diastereo- and enantioselectivities under supramolecular iminium catalysis which activates both the substrates and the reactive intermediates.


 Please wait while we load your content...
Please wait while we load your content...