C3-Selective alkenylation of N-acylindoles with unactivated internal alkynes by cooperative nickel/aluminium catalysis†
Abstract
Highly regio- and stereoselective alkenylation of N-acylindoles with unactivated internal alkynes has been accomplished by cooperative nickel/aluminium catalysis to afford C3-alkenylated indoles. Coordination of the acyl moiety to a bulky aluminium-based Lewis acid plays a crucial role in the selective functionalization at the C3-position by electron-rich nickel(0) catalysis.
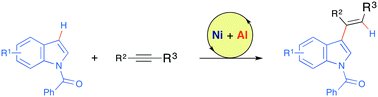


 Please wait while we load your content...
Please wait while we load your content...