Selective palladium(ii)-mediated oxidation of homoallylic N-tert-butanesulfinyl amine derivatives†
Abstract
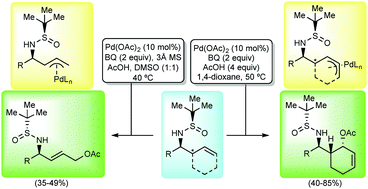
The palladium(II)-catalyzed oxidation of homoallylic amine derivatives resulting from the allylation of N-tert-butanesulfinyl imines with allyl bromide led to the formation of the corresponding terminal allylic acetates in a regioselective fashion in moderate yields. In the case of the homoallylic amine derivatives obtained using cyclohexenyl bromide as the allylating reagent, the allylic oxidation took place with high regio- and diastereoselectivity and yields ranging from 40 to 85%.

 Please wait while we load your content...
Please wait while we load your content...