Mixing cations with different alkyl chain lengths markedly depresses the melting point in deep eutectic solvents formed from alkylammonium bromide salts and urea†
Abstract
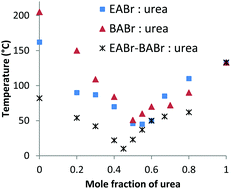
The melting point of a deep eutectic solvent formed from a ternary mixture of ethylammonium bromide (EABr), butylammonium bromide (BABr) and urea is 10 °C, which is almost 40 °C lower than the melting points of binary DESs formed from either EABr:urea or BABr:urea mixtures. This reveals a new route to prepare room temperature DESs via mixing different cations.


 Please wait while we load your content...
Please wait while we load your content...