Optically probing the localized to delocalized transition in Mo2–Mo2 mixed-valence systems†
Abstract
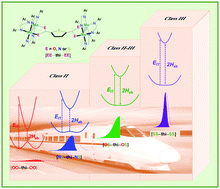
Four thienylene (C4H2S) bridged Mo2 dimers, [Mo2(DAniF)3]2(μ-OOCC4H2SCOO) (DAniF = N,N′-di(p-anisyl)formamidinate), [Mo2(DAniF)3]2(μ-N(H)SCC4H2SCN(H)S), [Mo2(DAniF)3]2(μ-OSCC4H2SCSO) and [Mo2(DAniF)3]2(μ-SSCC4H2SCSS), have been synthesized and studied in terms of electronic coupling. The subtle structural differences between these compounds vary largely the extent of electron delocalization; consequently, a systematic transition from Class II to Class III via Class II–III is achieved, which is probed using spectral parameters of intervalence charge transfer (IVCT) absorption (band energy, intensity and shape) for the mixed-valence complexes. Significantly, the species in Class II–III displays a low energy, half cut-off and solvent-dependent IVCT band, while a high energy, less asymmetrical IVCT band is observed for the complex in Class III. These results give fresh and detailed understanding of the system transition.


 Please wait while we load your content...
Please wait while we load your content...